What Best Describes the Heat Energy of the Boiling Water
In addition gas molecules leaving the liquid remove thermal energy from the liquid. As it falls the kinetic energy transforms into potential energy.
B They represent different concepts.

. Isaac wants to make smores. Question 2 30 seconds Q. Thermal energy from the fire moves to the water in the form of heat.
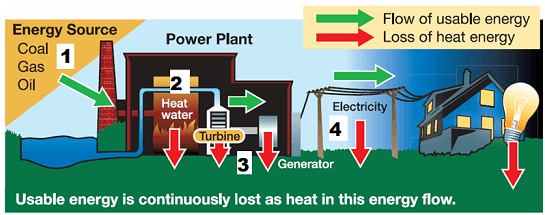
In these power plants the heat from burning coal is used to boil water. 2 What would best describe the boiling point. The walls of the beaker reflect heat toward the center so the water heats evenly.
Answer choices Burning coal and boiling water are both physical changes Burning coal and boiling water are both chemical changes. Boiling water takes more energy because more intermolecular forces are broken. Some energy is destroyed in the bounce resulting in a shorter bounce each time.
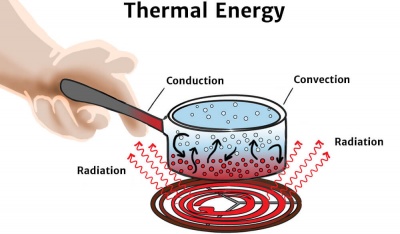
Which BEST describes how the thermal energy in the water and in. Heat from the burner radiates through the liquid and causes even heating. Yet this seemingly simple.
The stove transferred electricity to the pot of water. 5 What happens in a heating curve. As more energy goes into the matter the particles vibrate faster.
At some point on its path up there is 900 J of kinetic energy in the system. There are exactly. When the ball is dropped it has high kinetic energy and low potential energy.
As it falls the potential energy transforms into kinetic energy. The stove transferred mechanical energy to the pot of water. Heat energy is a measure of how fast particles of matter are vibrating.
Which statement best describes how heat energy is involved in changing water into steam. Which statement best describes heat and thermal energy. What causes the air above a pot of boiling water to become warm.
Which statement best describes the changes that occur when burning coal and boiling water. Why is boiling water a Heat Energy. Some energy is also transformed into sound and thermal.
A student standing near a campfire feels warmer as the fire grows. Which choice best describes the amount and form of energy in this system when the rock is at this point. Heat energy transferred from the rock to the water by.
The simple act of boiling water is one of humankinds oldest inventions and still central to many of todays technologies from coffee makers to nuclear power plants. What happens to the temperature of water while it is boiling. Melting ice or boiling water.
6 How do you find the boiling point of a graph. Answer choices The stove transferred light to the pot of water. Which best explains why water boils in a pot sitting over fire.
Which substance requires more heat to be warmed from room temperature to 50 C Water requires more heat because it has a higher specific heat. Water has a specific heat of 100 calg C and wood has a specific heat of 010 calg C. Heat energy breaks the intermolecular forces holding molecules of water together Which process requires more energy per gram.
3 How can you recognize the melting point and the boiling point in a heating curve. The boiling point of the water is reduced by the metal. The stove transferred heat energy to the pot of water.
The isolated system consists of the rock the person and the earth ignoring air resistance. A rock is thrown in the air with 1000 J of energy. Therefore the temperature of the liquid remains constant during boiling.
7 What is the. A They are different words for the same thing. 4 What type of lines show a temperature change on a heating curve.
1 How is the boiling process represented on a heating curve. A The air transfers thermal energy to the water vapor. Some power plants produce energy by burning coal.
Temperature and Boiling It requires energy to change from a liquid to a gas see enthalpy of vaporization. A hot rock is dropped into a pail of cool water. At some point it is.
B The water vapor transfers thermal energy to the air. Water boils when the thermal energy in the water which is a type of kinetic energy which causes the water molecules to move around exceeds the strength of the hydrogen bonds between the molecules causing them to separate from the other molecules. Evaporation heats the water at the top and the flame heats the water at the bottom.
Heat Thermal Energy And Temperature Quiz Quizizz


No comments for "What Best Describes the Heat Energy of the Boiling Water"
Post a Comment